Research at the Laboratory of Crystallography
Experimental static and dynamic electron densities of alpha-Glycine and D,L-Serine
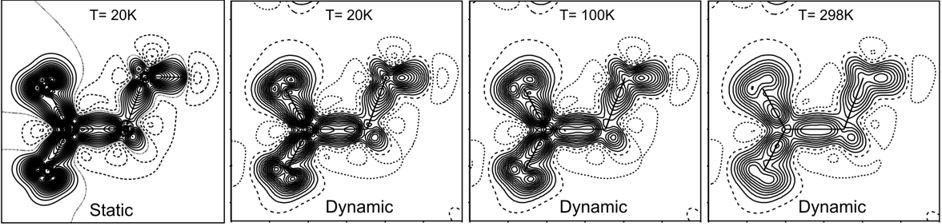
Dynamic electron densities can be obtained by the convolution of static electron densities and probability distribution functions of the individual atoms from an experimentally fitted electron density model [1]. A software "PRIOR" has been written with which dynamic electron densities can be constructed for both independent atom models and multipole (MP) models. The experimental static and dynamic electron densities of alpha-Glycine and D,L-Serine have been successfully computed for multipole models at temperatures 20, 100 and 298K. At temperatures below 100 K bond critical points (BCPs) and their properties are essentially the same for dynamic and static densities obtained from the MP model. At room temperature, larger differences have been found, especially in Laplacians at BCPs of polar covalent bonds. In spite of pronounced thermal smearing, essential bonding features are preserved in dynamic electron densities even at room temperature. Analysis of the experimental dynamic density will be helpful in revealing the effects of temperature on chemical interactions and properties of solids.
[1] S. Mondal, S.J. Prathapa, S. van Smaalen, Acta Cryst. A 68, 568 (2012)
